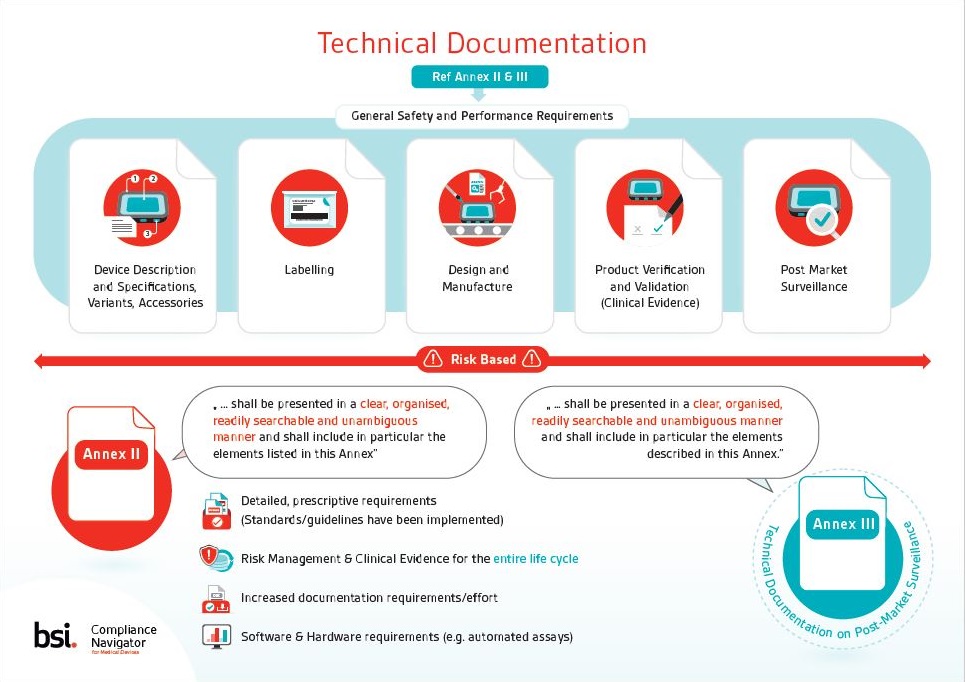
The Evolution of Medical Device Clinical Trials: Their Background and a Look Toward the Future, Free MasterControl Inc. White Paper

FDA-MITRE white paper prescribes next steps in managing legacy medical device cybersecurity risks - Industrial Cyber