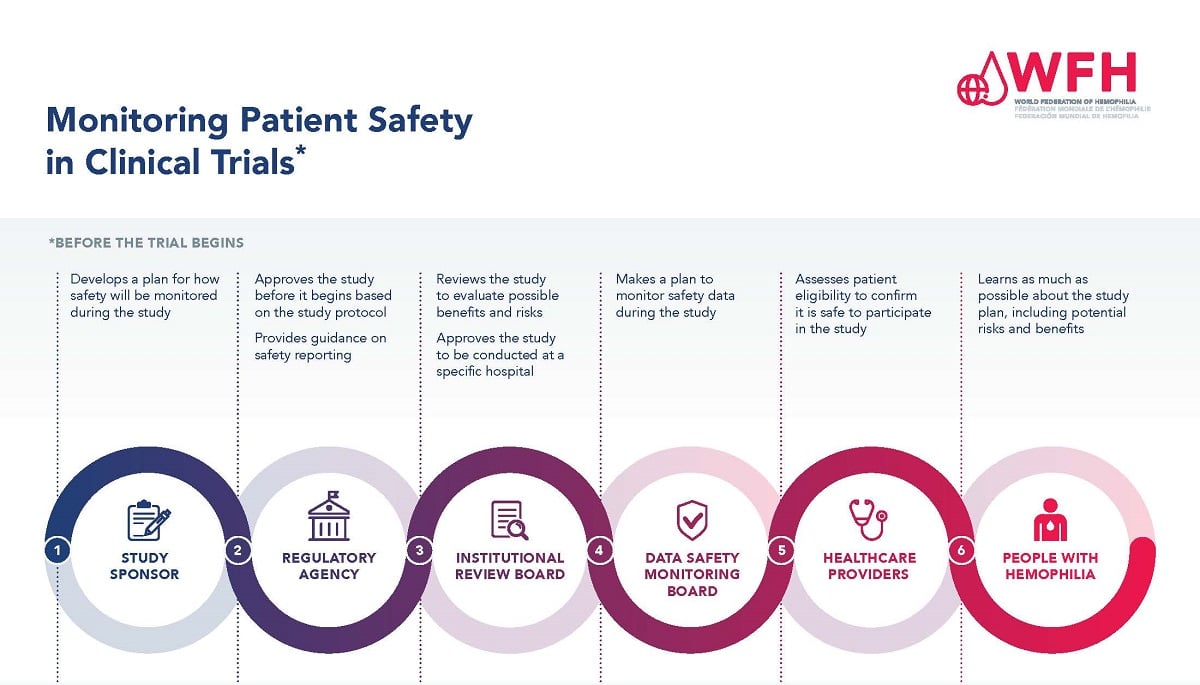
Guidelines for Developing a Data and Safety Monitoring Plan | National Institute on Drug Abuse (NIDA)

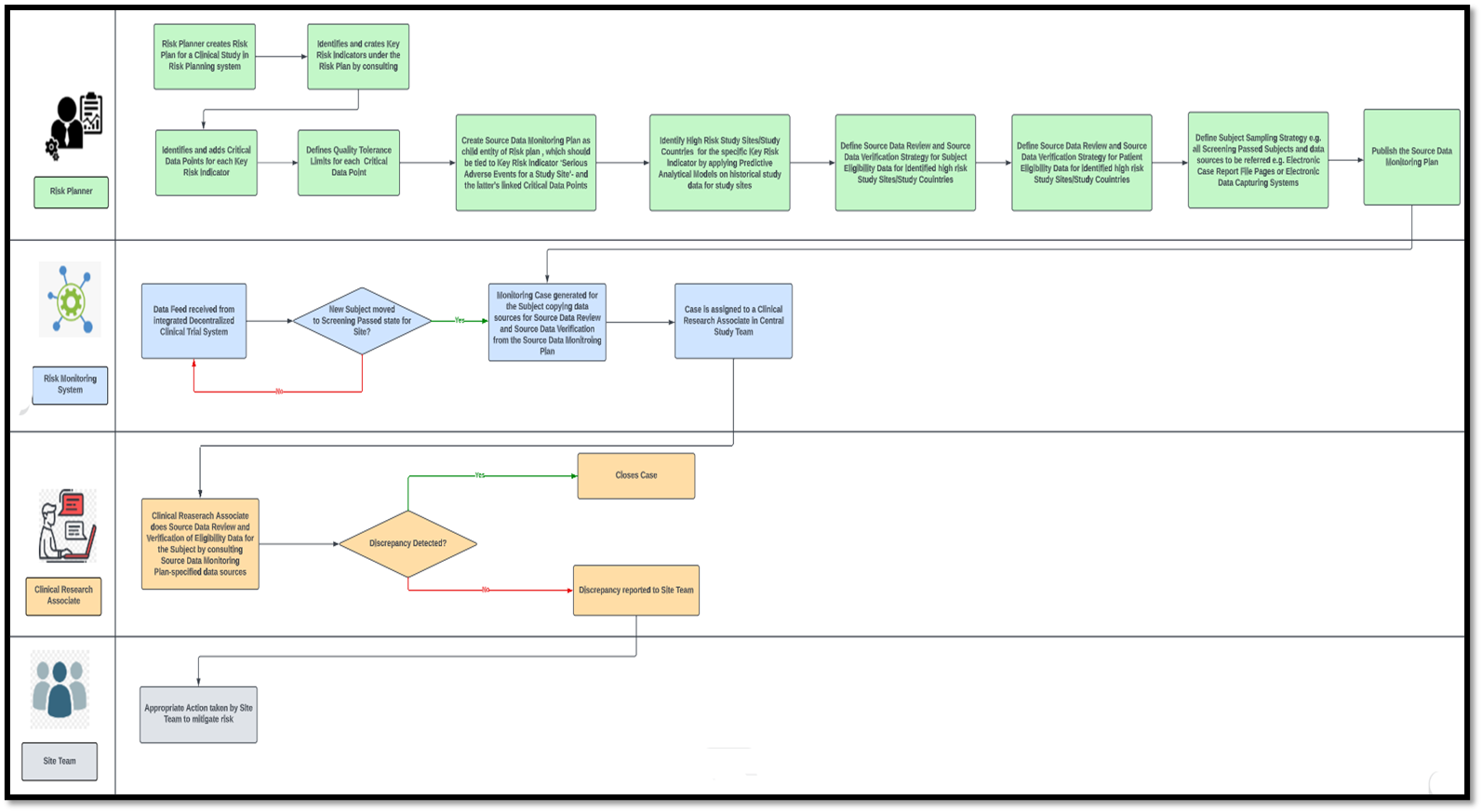
Validation of a risk-assessment scale and a risk-adapted monitoring plan for academic clinical research studies — The Pre-Optimon study - ScienceDirect

Trial Report Template (4) | PROFESSIONAL TEMPLATES | Report template, Report writing template, Writing templates

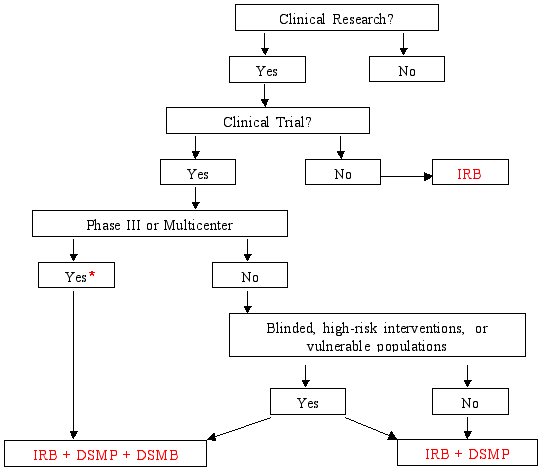
Figure 3 from Validation of a risk-assessment scale and a risk-adapted monitoring plan for academic clinical research studies--the Pre-Optimon study. | Semantic Scholar
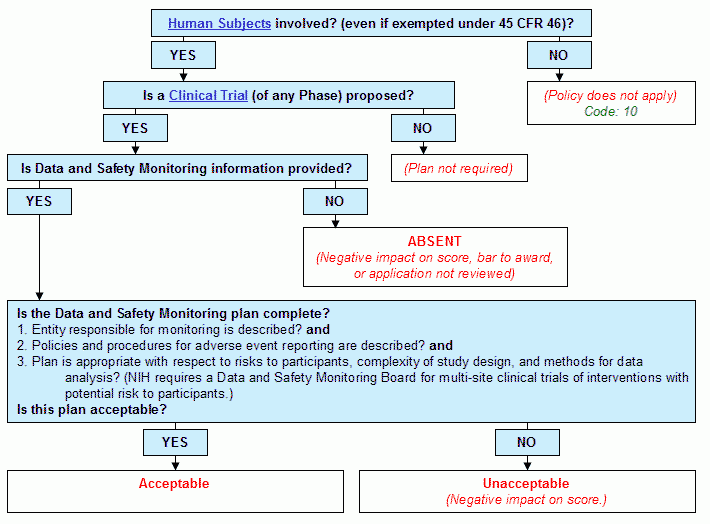
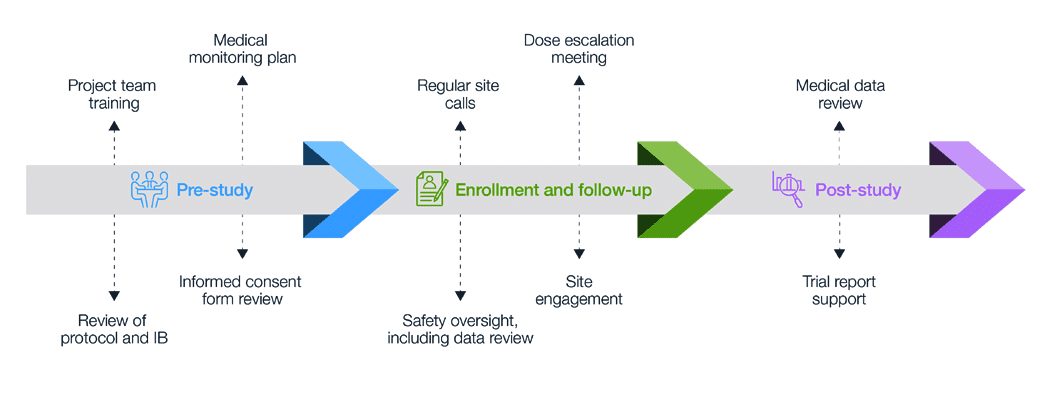
Decision Tree for Data and Safety Monitoring Plan | NIAID: National Institute of Allergy and Infectious Diseases

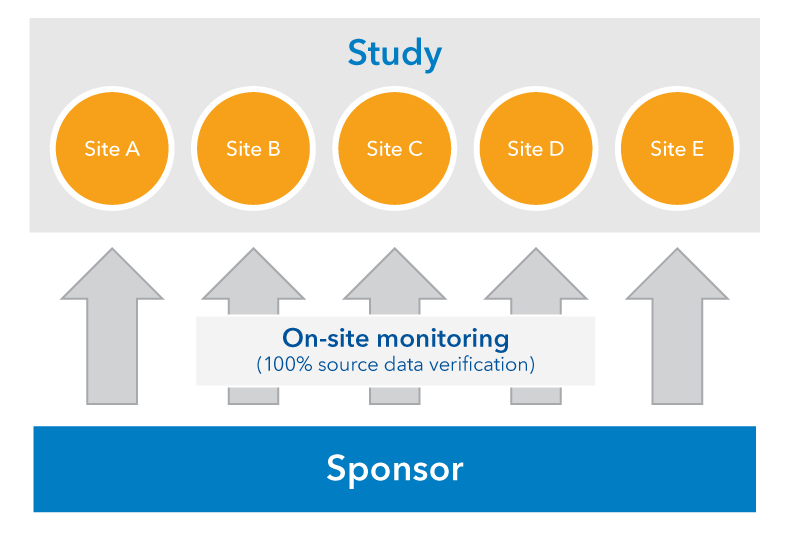
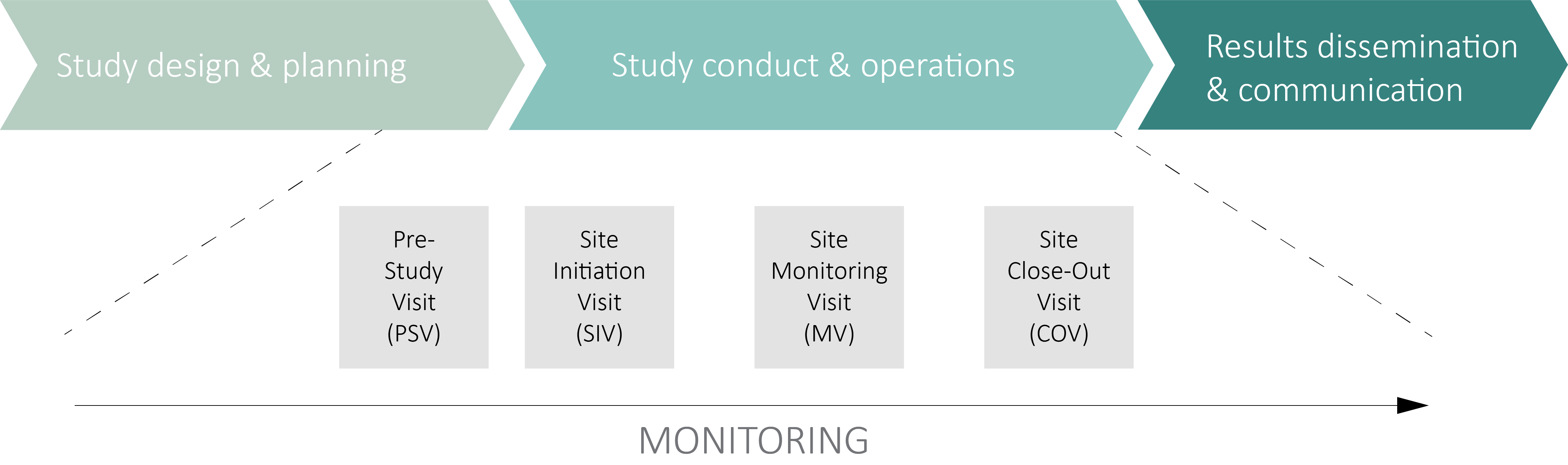
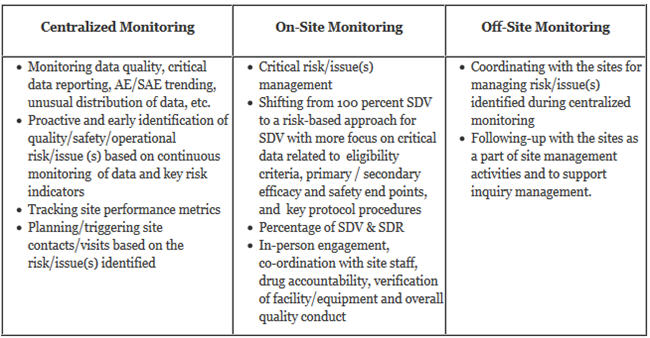
Risk-proportionate clinical trial monitoring: an example approach from a non-commercial trials unit | Trials | Full Text